- Case Report
- Open access
- Published:
A report of twenty cases of ovarian Brenner tumor and literature review: a case series study
BMC Women's Health volume 24, Article number: 471 (2024)
Abstract
Background
To explore the clinical characteristics of ovarian Brenner tumors and provide some basis for the treatment regimen of ovarian Brenner tumors.
Methods
A retrospective analysis of the pathology database of surgical specimens at the Huzhou Maternal and Child Health Hospital from September 2008 to February 2023 was conducted. Patients who were pathologically diagnosed with ovarian Brenner tumors were included. Clinical data of patients was collected, and their diagnostic and treatment characteristics were summarized and analyzed.
Results
A total of 20 cases were included in this study, all of which were histologically confirmed by surgical pathology. Among them, 8 cases (40%) were combined with serous, mucinous cystadenoma, or simple cyst. One case presented with a benign ovarian Brenner tumor combined with mucinous cystadenoma, underwent right adnexectomy, and relapsed 5 years later as a malignant Brenner tumor (MBT) coexisting with ovarian squamous cell carcinoma. Multiple tumor markers were elevated malignantly, with CA199 being the most significant. Treatments included unilateral adnexectomy in 7 cases, bilateral adnexectomy in 3 cases, total hysterectomy with bilateral adnexectomy in 7 cases, radical hysterectomy in 1 case, and 2 cases underwent ovarian staging surgery. MBT patients received three cycles of postoperative chemotherapy with the carboplatin-paclitaxel (TC) regimen. Follow-up: One case with concomitant cervical cancer was lost to follow-up after surgery in an external hospital; one case with concomitant ovarian cancer received no further treatment after surgery and was lost to follow-up after 2 years; one case with concomitant endometrial cancer received no further treatment after surgery, and had no recurrence after 4 years of follow-up. Regular follow-up for MBT patients continued for 5 years without recurrence. The remaining 16 cases were followed up for a period ranging from 6 months to 7 years, with no reported recurrences.
Conclusion
Clinical manifestations and auxiliary examinations of ovarian Brenner tumors lack obvious specificity. When necessary, a combination of tumor markers and imaging examinations can aid in diagnosis. Surgical strategies should be selected according to the patient's menopausal status.
Trial registration
Not applicable.
Background
Ovarian Brenner tumor is a rare neoplasm of the female reproductive system, accounting for approximately 2% to 3% of all ovarian tumors [1]. The World Health Organization classifies them as benign, borderline (proliferative), and malignant [2]. The majority of tumors are benign, with malignant tumors accounting for less than 5% of cases [3]. Approximately 85% of ovarian cancers are diagnosed with pelvic metastases, with a five-year survival rate of around 30% for these late-stage patients, compared to a 90% five-year survival rate for early-stage ovarian cancer [4]. Currently, ovarian Brenner tumors lack typical clinical, laboratory, or radiological features, making diagnosis challenging. They are often incidentally discovered, and existing studies both domestically and internationally are often limited to case reports and small retrospective studies, indicating a lack of comprehensive understanding and guidelines for this rare disease. Previous treatments for ovarian Brenner tumors have included surgical resection, adjuvant chemotherapy for malignant tumors, and radiotherapy for localized lesions. In this study, we conducted a retrospective analysis of the clinical data of 20 cases treated at our hospital over the past 15 years to investigate the clinical characteristics and treatment regimens of ovarian Brenner tumors, aiming to provide evidence for their clinical features and treatment strategies.
Methods
General information
A retrospective review was conducted on the pathological database of ovarian Brenner tumor patients' surgical specimens at the Huzhou Maternal and Child Health Hospital from September 2008 to February 2023. This study was a case series study, and there were no direct physical risks to the study patients.
Ethics
Prior to the commencement of the study, approval was obtained from the Huzhou Maternity and Child Health Care Hospital's ethics committee. This study was classified as a retrospective study and did not require informed consent from the subjects.
Inclusion criteria
Clinical Parameters of Patients: We retrospectively retrieved clinical and medical record data of patients, including age, menopausal status, clinical presentations, auxiliary examinations (laboratory test results and imaging findings), lesion characteristics (unilateral or bilateral, left or right), type of surgery, surgical indications, postoperative treatments, pathological results, and follow-up data, from electronic medical records.
Results
Clinical characteristics of brenner tumors (Table 1)
Clinical presentation: Ages ranged from 47 to 67 years, with a mean age of 58 years, and 18 cases occurred in postmenopausal women; among them, 19 cases were benign, and 1 case of benign tumor relapsed into malignancy after treatment (5%). Surgery was performed in 13 out of 20 cases due to pelvic masses or other reasons, and the diagnosis was incidentally discovered postoperatively. Abdominal distension was reported in 3 out of 20 cases (lesion diameter > 10 cm), and 1 out of 20 cases presented twice with "abdominal distension" at different times (lesion diameter > 10 cm). Three out of 20 cases had irregular vaginal bleeding (1 case with endometrial cancer, 1 case with cervical cancer); in 7 out of 20 cases, no ovarian lesions were detected preoperatively or intraoperatively, but were confirmed based on postoperative pathology. All patients were confirmed by surgical pathology. Nineteen out of 20 cases were unilateral (7 on the right side, 12 on the left side) and 1 out of 20 cases presented bilaterally at different times. Three cases were pure ovarian Brenner tumors, 8 cases (40%) were associated with serous or mucinous cystadenomas or simple cysts, 1 case was associated with cervical cancer, 1 case was associated with ovarian cancer, and 5 cases were associated with endometrial polyps, cervical lesions, and uterine fibroids. The three pure ovarian Brenner tumor lesions were solid on sectioning, with a grey-white or grey-yellow color and a hard texture. In four cases, grey-yellow or grey-white lesions were seen on sectioning of the ovaries, and in six cases, no obvious abnormalities were observed upon sectioning of the ovaries.
Ancillary Investigations: Preoperatively, all patients underwent routine gynecological examinations, tumor marker tests, and ultrasound examinations. Five patients underwent pelvic contrast-enhanced MRI, and ten patients underwent estradiol tests. Case 13's MRI showed a lump-like signal mass in the left adnexal area. The signal intensity was low on T1W1 and fs-T2W1, and there was no clear restricted diffusion on DWI. There was also some mild enhancement after contrast enhancement. Ultrasound examination (as shown in Fig. 1A) in Case 13 revealed hypoechoic areas in the left adnexal region with multiple strong echo spots (suggestive of ovarian fibroids) and significant posterior attenuation. Ultrasound findings in Cases 5 and 14 were similar to those in Case 13. In Case 10, a woman with cervical cancer, the MRI did not show any ovarian lesions. In the other three cases, the MRI showed cystic masses in the adnexal area that did not clearly enhance or only slightly enhanced after contrast enhancement. Seven cases showed no adnexal masses on ultrasound, and nine cases showed mixed echogenicity masses in the adnexal area. Initial ultrasound in Case 20 revealed multilocular cystic masses in the pelvis with no blood flow signal, while recurrent ultrasound (as shown in Fig. 1B and C) showed predominantly solid masses in the pelvis with abundant blood flow signals. Preoperatively, tumor markers were normal in 15 cases, and four cases showed elevated squamous cell carcinoma-related antigen (SCC). SCC levels were elevated in Cases 3 (cervical CINIII), 10 (cervical cancer), 13, and 20 at both initial and recurrent presentations (Table 2), which returned to normal postoperatively. Multiple tumor markers were elevated in Case 20 at the time of malignant onset, as detailed in the typical case. Carbohydrate antigen 153 was elevated preoperatively in Case 15 (Table 2), which returned to normal four days postoperatively. Estradiol tests were performed in 10 cases, all of which were postmenopausal patients. Eight cases were within the normal range, Case 19 (endometrial irregular hyperplasia), and the initial presentation of Case 20 showed elevated levels (Tables 2 and 3), which remained unchecked postoperatively.
Imaging Images of Brenner's Tumor. A Case 13: there was a hypoechoic area measuring, with multiple strong echogenic spots observed within it. Significant posterior acoustic shadowing was evident. B Case 20 MBT Ultrasound: A predominantly solid mixed echogenic mass was observed in the pelvic cavity. C Case 20 MBT Ultrasound: Abundant blood flow signals were observed within the mixed echogenic mass. D Case 20 MBT Ultrasound: Abundant blood flow signals were observed within the mixed echogenic mass. E Case 20 MBT CT Plain Scan: A large lobulated cystic-solid mass was observed in the pelvic cavity. The border appeared smooth, with multiple septations seen within. F Case 20 MBT Enhanced CT: Post-contrast imaging revealed significant enhancement of the solid components
Treatment: Seven cases underwent unilateral adnexectomy and three cases underwent bilateral adnexectomy. Seven cases underwent total hysterectomy + bilateral adnexectomy. One case underwent radical hysterectomy for cervical cancer, and two cases underwent ovarian staging surgery. Three cycles of chemotherapy with the TC regimen were administered postoperatively in cases of MBTs.
Prognosis: One case with cervical cancer received external radiotherapy postoperatively at an outside hospital and was lost to follow-up. One case with ovarian cancer received no further treatment postoperatively and was lost to follow-up after two years. One case with endometrial cancer received no further treatment postoperatively and remained recurrence-free after four years of follow-up. Regular follow-up for five years showed no recurrence in patients with MBTs. The remaining 16 cases were followed up for 6 months to 7 years postoperatively, with no recurrence observed.
Typical clinical characteristics of case 20 (Table 4)
The patient, aged 54 in 2012, had been postmenopausal for 4 + years when she presented to our hospital with complaints of "lower abdominal distension for over a month, abdominal pain for 2 days." Upon admission, gynecological examination revealed a cystic mass measuring approximately 15 cm in diameter within the pelvic cavity, with a palpation tenderness of + + and suspected rebound tenderness. Ultrasonography indicated a low hypoechoic nodule in the uterus, endometrial thickness of 4 mm, and multiple multilocular cystic masses within the pelvic cavity (148*125*120 mm, likely of adnexal origin). E2 levels were measured at 154.2 pmol/L (laboratory reference postmenopausal < 100 pmol/L), tumor marker SCC at 2.7 ng/ml (normal range < 1.5 ng/ml), and cervical liquid-based cytology revealed inflammatory reactive changes with positive HPV-DNA. Ovarian tumor was suspected with torsion not excluded. Emergency exploratory laparotomy and left adnexectomy were performed, revealing a cyst approximately 15*14*12 cm in size in the pelvic cavity of the left ovarian origin. The pedicle and left fallopian tube were twisted 90 degrees, showing a red pedicle and fallopian tube without obvious necrosis. The right ovary was atrophic with no apparent abnormalities, and the right fallopian tube appeared normal. The uterus was of normal size with a smooth surface, and no significant fluid accumulation was observed in the pelvic and abdominal cavities. Postoperative pathology indicated (left) Brenner tumor combined with mucinous cystadenoma. Immunohistochemistry showed CA125 (-), CD99 (-), CKpan ( +), Inhibin-a (-), and Ki-67 (+ 10%). Postoperatively, SCC decreased to 0.4 ng/ml.
In 2017, at the age of 59, the patient presented with complaints of "abdominal distension for 2 months" and underwent pelvic enhanced CT scan at an external hospital (refer to Fig. 1D, E): a large lobulated cystic-solid mass measuring approximately 156 mm*121 mm was observed in the pelvic cavity with smooth borders and multiple septations internally. Contrast-enhanced scans and solid components showed significant enhancement. The bladder and uterus were compressed, with unclear demarcation from the anterior wall of the uterus, and no bladder filling was observed. A small amount of fluid was present in the pelvic cavity, and no enlarged lymph nodes were noted. The imaging suggested a pelvic mass, considering cystadenocarcinoma, invasion of the anterior wall of the uterus, and a small amount of pelvic fluid accumulation. Malignant ovarian tumor was considered, and surgery was recommended, but the patient did not undergo treatment at the external hospital and instead sought immediate consultation at our hospital, where she was admitted. Upon admission, gynecological examination revealed a 15*12*10 cm solid mass behind the uterus with poor mobility and no tenderness. Ultrasound examination showed a predominantly solid mixed echogenic mass measuring 132*117*93 mm in the pelvic cavity, with poor sonolucency within the cystic area, abundant blood flow signals on color Doppler flow imaging (CDFI), and an RI of 0.38–0.45, indicating a mixed echogenic mass in the pelvic cavity (likely ovarian cancer with invasion of the uterine body, most likely originating from the right ovary), and pelvic and abdominal fluid accumulation. Preoperative levels of CA199 were 1530.57 IU/ml (normal range ≤ 37 U/ml), CA125 was 63.54 IU/ml (reference normal range ≤ 35 U/ml), CA153 was 38.2 IU/ml (normal range ≤ 20 U/ml), SCC was 17.7 ng/ml (normal range < 0.5 ng/ml), CEA was 26.43 ng/ml (normal range ≤ 5 ng/ml), and estradiol was 39.4 pmol/L. Cervical liquid-based cytology at the external hospital was normal, and HPV testing was not performed. Considering "malignant ovarian tumor," the patient underwent ovarian cancer staging surgery (total hysterectomy + right adnexectomy + bilateral ovarian artery ligation + pelvic lymph node dissection + para-aortic lymph node biopsy + omentectomy + multiple peritoneal biopsies). Intraoperatively, a small amount of pale-yellow fluid was found in the pelvic cavity, and exploration revealed no obvious abnormalities on the surface of the liver, gallbladder, pancreas, spleen, omentum, diaphragm, or intestinal surfaces. The transverse colon was densely adhered to the left abdominal wall, and the rectal sigmoid colon was adhered to the anterior abdominal wall. The remaining intestinal tract and appendix showed no abnormalities, and the ureter and bladder were normal. Pelvic lymph nodes and para-aortic lymph nodes were not enlarged. The uterus was anteverted, slightly smaller, with a smooth surface, soft bilateral parametrial tissues, normal uterosacral ligaments and sacroiliac ligaments, and a slightly irregularly enlarged right ovary measuring 15*12*9 cm, with a grey white color and moderate texture. The surface of the right fallopian tube was seen lying on the clinical surface without obvious growths, and the left adnexa was absent. Intraoperative slicing revealed a cystic-solid appearance with grey-white and grey yellow colors. Rapid pathology during surgery indicated malignant tumor of the right ovary, considered squamous cell carcinoma. Postoperative pathology confirmed MBT (including squamous cell carcinoma component) of the right ovary, fallopian tube tissue (left), uterine leiomyoma, endometrial atrophy, chronic inflammation of the cervical mucosa with phosphitylation, adipose connective tissue (omentum), adipose connective tissue (left colonic sulcus, left rectal sulcus, right colonic sulcus, right rectal sulcus, adhesions of the rectosigmoid colon), and 22 lymph nodes submitted for examination showed no metastasis. Immunohistochemistry results were as follows: ER (-), PR (-), P53 ( +), HCK (34βH11) (-), WT1 (-), CK7 ( +), CK20 (-), Ki-67( +) 20%, CEA ( +), and P16 (-). Pathological consultation was conducted at the Chinese Academy of Sciences University Affiliated Cancer Hospital (Zhejiang Cancer Hospital), confirming our hospital's findings. Postoperative staging was determined as stage Ia MBT of the ovary (moderately differentiated), and the patient received three cycles of TC regimen chemotherapy postoperatively. After one cycle of chemotherapy, all tumor markers returned to normal levels. No recurrence was observed during the 5-year follow-up postoperatively.
Discussion
Ovarian Brenner tumors can occur at any age, with a higher prevalence among postmenopausal women, affecting approximately 80% of patients over 50 years old. Benign alterations typically manifest unilaterally, with a predilection for the left ovary. Approximately 5% to 14% of ovarian MBTs present are bilateral, and the risk of malignancy increases with age among women with ovarian Brenner tumors [5]. The findings of this study are consistent with these observations. MBTs represent a highly uncommon subtype of ovarian epithelial tumors. The precise incidence of MBTs remains unclear, with existing research primarily comprising case reports and small-scale case studies. Conversely, primary ovarian squamous cell carcinoma is exceedingly rare, accounting for only 0.5% of ovarian cancers. In the 2020 WHO classification, ovarian squamous cell carcinoma is classified as a somatic-type tumor originating from teratomas. As the majority of cases arise from the malignant transformation of mature cystic teratomas, accounting for 80% of cases of teratoma malignant transformation [6, 7], with a minority possibly developing from Brenner tumors [8]. Studies have found an association between HPV infection and reproductive system tumors, particularly cervical cancer. Additionally, Svahn et al. [9] found that 15% of ovarian epithelial cancers were associated with HPV infection, with a higher prevalence in Asian regions. The carcinogenicity of HPV has been confirmed in various tumors, with recent research suggesting that HPV establishes persistent infection through the post-infection microenvironment, thereby inducing cervical epithelial dysplasia [10]. In the present case, the patient had a history of HPV infection and Brenner tumor, suggesting two factors associated with the development of ovarian squamous cell carcinoma. Combining the aforementioned literature, this case report may provide a basis for studying the reasons for the occurrence of ovarian squamous cell carcinoma.
Current research suggests that the clinical symptoms of ovarian Brenner tumors are similar to those of other epithelial ovarian tumors, with the most common being abdominal mass and distension, followed by abdominal pain and postmenopausal vaginal bleeding. Other symptoms may include nausea, vomiting, dyspepsia, and constipation, while some patients may be asymptomatic [11]. Combining the findings of this study indicates that the clinical symptoms of ovarian Brenner tumors lack distinct specificity and are associated with patient comorbidities, tumor size, and malignancy. Approximately 25% to 36% of Brenner tumors may be associated with other benign ovarian tumors, such as serous cystadenoma, mucinous cystadenoma, and teratoma, possibly due to hormone secretion by Brenner tumor cells, leading to local cellular proliferation [12]. In this study, 40% of cases were associated with other benign ovarian cysts, slightly higher than reported in the literature, although the sample size was small and requires extensive data analysis to verify. Ovarian malignant tumors are often challenging to detect in early stages, with over 85% of patients diagnosed at advanced stages. The five-year survival rate for early-stage lesions can reach 90% [4]. However, a survey conducted in Italy indicated a decrease in quality of life and changes in sexual activity among gynecological cancer survivors [13], emphasizing the critical importance of early detection and treatment of ovarian lesions. The lack of distinct clinical symptoms in ovarian Brenner tumors often leads to delayed diagnosis, with clinical symptoms frequently attributed to other conditions, necessitating postoperative pathological diagnosis. In this study, 13 out of 20 cases underwent surgical treatment due to pelvic masses or other reasons, with Brenner tumors incidentally discovered postoperatively.
Regarding tumor markers, there are currently no specific indicators. It has been reported that approximately 70% of patients with MBTs exhibit elevated levels of Cancer Antigen 125 (CA125), which gradually decrease to normal levels after treatment and increase again after recurrence [5, 14]. However, in this study, CA125 levels were normal in all 19 cases of benign Brenner tumors. Three cases of benign Brenner tumors showed elevated levels of Squamous Cell Carcinoma Antigen (SCC), excluding one case of cervical cancer, and one case showed an increase in Carbohydrate Antigen 153 (CA153). In case 20, when malignancy occurred, CA125 and CA153 levels were slightly elevated, while SCC and Carcinoembryonic Antigen (CEA) levels were significantly elevated, with the most significant increase observed in CA199. CA199 is typically synthesized by normal glandular epithelial cells, such as pancreatic and bile duct cells, as well as epithelial cells of the stomach, colon, endometrium, bronchi, and salivary glands. Clinically, elevated levels of CA199 are initially associated with digestive system diseases, particularly malignant tumors. CA199 levels are elevated in malignant ovarian tumors, especially mucinous ovarian tumors, although the diagnosis of ovarian tumors lacks specificity [15, 16]. Qian et al. [17] reported the first case of abnormally high serum CA199 levels in a transitional Brenner tumor in 2022. This study also presents a unique case of significant CA199 elevation, although the patient had concomitant ovarian squamous cell carcinoma, suggesting a possible association. Chiang et al. [18] demonstrated that SCC, CA125 and CEA, among the tumor markers of primary ovarian squamous cell carcinoma, were elevated in the peripheral blood of many patients, which were related to the therapeutic effect and prognosis of the disease. Case 20 supports this view, indicating that if CA125, CEA, and SCC Ag levels are elevated, the possibility of ovarian squamous cell carcinoma should be considered. When CA199 levels are abnormally elevated without reasonable explanation, the possibility of ovarian Brenner tumor should also be considered. Combining three cases of elevated SCC and one case of elevated CA153, which normalized postoperatively, whether SCC and CA153 can assist in diagnosing Brenner tumors and warrants further investigation.
The typical ultrasound appearance of ovarian Brenner tumors is termed the "shell sign" [19] characterized by a strong echogenic mass in the anterior part of the tumor with marked sound attenuation in the posterior part, but no obvious blood flow signal within the mass. In this study, the ultrasound findings of three cases of simple ovarian Brenner tumors closely resembled the typical shell sign (see Fig. 1A), although this appearance can also occur in other solid ovarian tumors. Our ultrasound findings suggested the possibility of fibroma or teratoma, lacking specificity. After contrast-enhanced CT imaging, it has been reported that benign ovarian Brenner tumors show mild enhancement, while lesions of MBTs exhibit moderate to high enhancement [20]. In this study, ultrasound and contrast-enhanced CT imaging of case 20 at malignancy presentation displayed similar features to other epithelial tumors (see Fig. 1B-E). Additionally, four cases of benign ovarian Brenner tumors underwent pelvic-enhanced MRI, with no apparent blood flow signal observed and no significant enhancement or only mild enhancement after contrast, indicating that ultrasound, contrast-enhanced CT, or enhanced MRI cannot provide specificity for diagnosing Brenner tumors but can serve as reference indices for distinguishing between benign and malignant tumors.
Reportedly, Brenner tumors exhibit hormonal activity, with oestrogen considered the primary hormone secreted by these tumors [21]. This, in turn, may lead to endometrial pathologies such as endometrial hyperplasia, occurring in 4% to 14% of cases [22]. In this study, only 2 cases showed elevated oestrogen levels, yet they presented with concomitant endometrial carcinoma, irregular endometrial hyperplasia, endometrial polyps, and abnormal uterine bleeding, suggesting that ovarian Brenner tumors may cause oestrogen abnormalities, thereby leading to endometrial-related diseases.
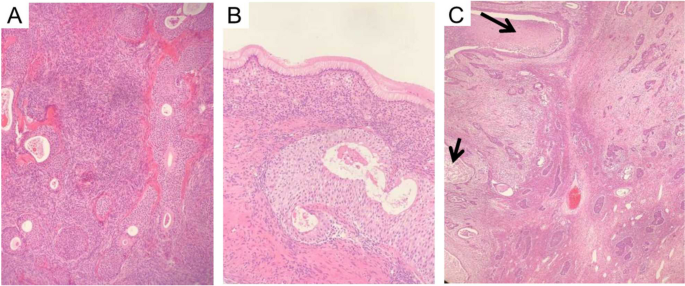
All cases underwent pathological diagnosis. A typical case, Case 20, first presented in 2012 with a benign Brenner tumor on the left side. It recurred and evolved into MBT in 2017, five years later. Our hospital's pathology department reflected on whether malignant components were missed in 2012 and reviewed the slides from that year. The distinction between benign and malignant ovarian Brenner tumors lies in the degree of cellular atypia and the presence of stromal infiltration. Benign Brenner tumors of the ovary typically consist of solid components, with tumor cells forming oval or irregular nests scattered within fibrous stroma. Nutrient-poor calcification and glassy changes can be observed within, with cell nuclei being oval or round, showing no obvious atypia [23]. Ovarian MBT histological characteristics include increased layers of tumor cell nests (Fig. 2A, B), deeply stained cell nuclei, polymorphism, and evident nuclear division activity (Fig. 2C), with the appearance of pathological mitotic figures and stromal infiltration [23]. Several related gene mutations may be associated with ovarian MBT, inducing the gradual progression of benign ovarian Brenner tumors to ovarian MBT [24, 25]. Therefore, gene mutations are associated with deterioration, and genetic testing may be considered in subsequent treatment.
Pathological picture of Case 20. A Case 20, 2012 Benign Brenner Tumor: Within the dense fibrous stroma of the ovary, oval or round-shaped nests of transitional cells were observed, exhibiting mild cellular morphology. Some nests showed cystic changes, lined with mucinous epithelium, with eosinophilic material seen in the center of the cysts. Magnification: 100x. B Case 20, 2012 Benign Brenner Tumor: At the lower end of the transitional cell nests, tall columnar mucinous epithelium was visible. Magnification: 400x. C Case 20, 2017 MBT with Squamous Cell Carcinoma Component: The epithelial nests of the tumor were irregular, with partial cystic dilatation on the left side containing abundant necrotic material. The epithelial cells exhibited marked anisocytosis, prominent nucleoli, and focal keratinization. Inter-cellular bridges were observed, indicative of squamous cell carcinoma (indicated by arrows). The nests on the right side varied in size, with irregular margins, crowded cells showing marked anisocytosis, increased nuclear-cytoplasmic ratio, thickened nuclear membranes, prominent nucleoli, and visible mitotic figures. In the background ovarian stroma, infiltration of acute and chronic inflammatory cells was observed, consistent with MBT. Magnification: 40x
The primary approach for treating ovarian Brenner tumors remains surgery. Due to the patients' advanced age, often in perimenopause or postmenopausal, adnexectomy serves as the main treatment for benign Brenner tumors, effectively reducing recurrence rates [26]. Similar to other epithelial ovarian cancers, the initial treatment for MBT is tumor-reducing surgery. In our study, MBT underwent standard staging surgery for ovarian cancer, but the role of lymph node dissection (LND) in this rare cancer subtype remains unclear. Recent research indicates no significant difference in disease specificity or survival rates between patients who underwent LND and those who did not, with only 5% of patients who underwent lymph node sampling showing lymph node metastasis [12]. Zhang et al. [3] also noted that 60% of ovarian MBT patients presented radiological evidence of lymph node involvement, but after lymph node dissection, the final pathological results showed negative lymph nodes, suggesting a lower likelihood of regional lymphatic spread in ovarian MBT. Therefore, the necessity of lymph node dissection in such diseases remains uncertain.
The role of adjuvant chemotherapy in MBT remains controversial. Gezginç et al. [27]. found that 90% (9/10) of patients receiving the TC regimen achieved complete remission, while 70% (7/10) of advanced-stage patients experienced recurrence after an average of 23.8 months following the aforementioned chemotherapy. Additionally, Yüksel et al. [5] found that all (7/7) patients treated with the platinum-taxane regimen achieved complete remission, with two stage III C patients experiencing recurrence at 86 months and 13 months after chemotherapy, respectively. Therefore, the platinum-taxane regimen as adjuvant chemotherapy demonstrates certain survival advantages post-surgery and may be used as first-line adjuvant chemotherapy for ovarian MBT, despite a high recurrence rate in advanced-stage patients. Regarding radiotherapy, first-line therapy is not recommended. A recent population-based analysis based on SEER reported that 2.4% of MBT patients received radiotherapy during treatment [14]. The postoperative treatment regimen for ovarian squamous cell carcinoma still lacks a unified standard. Based on years of treatment experience and retrospective data studies, most scholars recommend first-line treatment based on a TC regimen [28, 29]; for radiotherapy, some scholars believe that its side effects are significant and unfavorable for prognosis [6]. Through the cases in this study, the TC regimen further demonstrates survival advantages for early-stage MBT patients.
For ovarian MBT, a study based on the SEER database suggested that patients in stages I, II, III, and IV accounted for 55.4%, 14.4%, 18%, and 12.2% of the total number of ovarian MBT patients, respectively. Tumor staging is the most important predictor of disease-specific survival (DSS), with a 5-year DSS of 94.5% for stage I patients, decreasing to 51.3% if ovarian spread occurs [6]. Therefore, early detection and treatment are crucial. Combining the results of this study, Brenner tumors predominantly occur after menopause, with unilateral occurrence being common. The diagnosis of this disease is challenging based on clinical presentation and auxiliary examinations, leading to a certain delay in diagnosis and a certain recurrence rate. There is even a possibility of malignant development after recurrence. Therefore, for postmenopausal patients diagnosed with Brenner tumors, bilateral oophorectomy is recommended where feasible to reduce recurrence and the potential for malignant progression.
Conclusion
In conclusion, the clinical presentation and auxiliary examinations of ovarian Brenner tumors lack obvious specificity, and tumor markers and imaging examinations may be used to assist in diagnosis when necessary. Surgical strategies should be selected based on the patient's menopausal status.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- MBT:
-
Malignant Brenner tumor
- SCC:
-
Squamous cell carcinoma-related antigen
- CDFI:
-
Color Doppler flow imaging
References
Turgay B, Koyuncu K, Taşkın S, Ortaç UF. Features of ovarian Brenner tumors: Experience of a single tertiary center. Turk J Obstet Gynecol. 2017;14:133–7.
Parcesepe P, Coppola L, Remo A, D’Andrea MR, Coppola G, Simbolo M, et al. Molecular and Clinical Insights in Malignant Brenner Tumor of the Testis With Liver Metastases: A Case Report. Front Oncol. 2021;11:663489.
Zhang Y, Staley SA, Tucker K, Clark LH. Malignant Brenner tumor of the ovary: Case series and review of treatment strategies. Gynecol Oncol Rep. 2019;28:29–32.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Yüksel D, Kılıç C, Çakır C, Kimyon Cömert G, Turan T, Ünlübilgin E, et al. Brenner tumors of the ovary: clinical features and outcomes in a single-center cohort. J Turk Ger Gynecol Assoc. 2022;23:22–7.
Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, Münstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008;9:1173–80.
Chen RJ, Chen KY, Chang TC, Sheu BC, Chow SN, Huang SC. Prognosis and treatment of squamous cell carcinoma from a mature cystic teratoma of the ovary. J Formos Med Assoc. 2008;107:857–68.
Woodruff JD, Dietrich D, Genadry R, Parmley TH. Proliferative and malignant Brenner tumors. Review of 47 cases. Am J Obstet Gynecol. 1981;141:118–25.
Svahn MF, Faber MT, Christensen J, Norrild B, Kjaer SK. Prevalence of human papillomavirus in epithelial ovarian cancer tissue. A meta-analysis of observational studies. Acta Obstet Gynecol Scand. 2014;93:6–19.
Yuan Y, Cai X, Shen F, Ma F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021;497:243–54.
Singh BK, Saha S, Agarwal S, Rathore YS. Malignant Brenner tumour of the ovary manifesting as distal intestinal obstruction and perforation. BMJ Case Rep. 2020;13(6):e235394. https://doiorg.publicaciones.saludcastillayleon.es/10.1136/bcr-2020-235394.
Pavlovic A, Glavina Durdov M, Lozic D, Skare Librenjak L, Alfirevic D. Primary ovarian lymphoma and benign Brenner tumor. Taiwan J Obstet Gynecol. 2016;55:138–9.
La Spina S, Scollo P, Pecorino B, Lombardo V, Motta A, Calderone RG, et al. Life Experience of Survivors of Gynecologic Cancers: A Survey Conducted in Italy. Oncology (Williston Park). 2024;38:15–9.
Nasioudis D, Sisti G, Holcomb K, Kanninen T, Witkin SS. Malignant Brenner tumors of the ovary; a population-based analysis. Gynecol Oncol. 2016;142:44–9.
Salibay CJ, Zanfagnin V, Miller H, Walia S, Brunette LL, Wang T. Borderline Brenner Tumor of the Ovary Coexisting With an Ovarian Mucinous Cystadenoma With Focal Atypical Epithelial Proliferation: A Rare Case With Review of the Literature. Int J Surg Pathol. 2021;29:788–93.
Zhong P, Zhu L, Zhang L. Clinicopathological features of ovarian Brenner tumors. Zhonghua Bing Li Xue Za Zhi. 2019;48:615–9.
Yu Q, Zhao Q, Su Y, Xiong K, Lu Y, Zhang L, et al. Borderline Brenner tumor with abnormally high serum level of carbohydrate antigen 199: a rare case report and literature review. Ir J Med Sci. 2023;192:2071–5.
Chiang AJ, Chen DR, Cheng JT, Chang TH. Detection of human papillomavirus in squamous cell carcinoma arising from dermoid cysts. Taiwan J Obstet Gynecol. 2015;54:559–66.
Dierickx I, Valentin L, Van Holsbeke C, Jacomen G, Lissoni AA, Licameli A, et al. Imaging in gynecological disease (7): clinical and ultrasound features of Brenner tumors of the ovary. Ultrasound Obstet Gynecol. 2012;40:706–13.
Zhao Y, Mao X, Yao L, Shen J. Computed tomography imaging features of benign ovarian Brenner tumors. Oncol Lett. 2018;16:1141–6.
Chia CC, Huang SC. A borderline ovarian Brenner tumor mimicks uterine fibroids. Taiwan J Obstet Gynecol. 2011;50:103–5.
Akman L, Akdemir A, Terek MC, Zekioglu O. Ovarian malignant Brenner tumor in patients over 65 years of age. Kaohsiung J Med Sci. 2014;30:159–60.
Zhong P, Zhu L, Zhang L. Clinical and pathological analysis of ovarian Brenner tumors. Chinese Journal of Pathology. 2019;48:615–9.
Lang SM, Mills AM, Cantrell LA. Malignant Brenner tumor of the ovary: Review and case report. Gynecol Oncol Rep. 2017;22:26–31.
Toboni MD, Smith HJ, Dilley SE, Novak L, Leath CA. Malignant Brenner tumor associated with a germline BRCA2 mutation. Gynecol Oncol Rep. 2017;21:17–9.
Uzan C, Dufeu-Lefebvre M, Fauvet R, Gouy S, Duvillard P, Darai E, et al. Management and prognosis of borderline ovarian Brenner tumors. Int J Gynecol Cancer. 2012;22:1332–6.
Gezginç K, Karatayli R, Yazici F, Acar A, Çelik Ç, Çapar M, et al. Malignant Brenner tumor of the ovary: analysis of 13 cases. Int J Clin Oncol. 2012;17:324–9.
Ohtani K, Sakamoto H, Masaoka N, Shimada K, Kanaeda T, Kurihara M, et al. A case of rapidly growing ovarian squamous cell carcinoma successfully controlled by weekly paclitaxel-carboplatin administration. Gynecol Oncol. 2000;79:515–8.
Yazawa H, Hiraiwa T, Ito F, Fujimori K. Long-term recurrence-free survival of a patient with advanced pure primary ovarian squamous cell carcinoma treated with dose-dense paclitaxel combined with carboplatin. Obstet Gynecol Sci. 2017;60:587–92.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
LZX and MLN carried out the studies, participated in collecting data, and drafted the manuscript. WZQ performed the statistical analysis and participated in its design. GJL and ZWW participated in acquisition, analysis, or interpretation of data and draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
I confirm that all methods were performed in accordance with the relevant guidelines. This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study has been approved by the Medical Ethics Committee of Huzhou Maternity &Child Health Care Hospital (No. 2023-J-129), The requirement for informed consent was waived by the Institutional Review Board of Huzhou Maternity &Child Health Care Hospital because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lou, Z., Mei, L., Wan, Z. et al. A report of twenty cases of ovarian Brenner tumor and literature review: a case series study. BMC Women's Health 24, 471 (2024). https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12905-024-03316-4
Received:
Accepted:
Published:
DOI: https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12905-024-03316-4